The significance of cold chain engineering has taken recent prominence in part due to focus on the Covid-19 pandemic and the need for shipment of vaccines around the world. Transporting lifesaving and sensitive material can be challenging especially with the changing scope of the supply chain regulatory paradigm.
Various materials are currently being used to develop cold chain solutions for packaging. Phase change materials (PCMs) — which are materials that have the ability to store and release a large amount of heat/energy while maintaining a constant temperature — have delivered a new wave of innovative packaging products to the market. PCMs that release or absorb sufficient energy during phase transitions provide the required heating and cooling that is critical in cold chain management.
There is already a plethora of players currently involved in providing solutions using different phase change materials. In this article, we discuss how to select the phase change solutions fit for your product characteristics.
Understanding PCMs
A cold chain packing solution can be best described as the method of packaging and shipping a product to maintain a consistent temperature from end to end within a transit route, be it international or domestic in nature. PCM packing solutions currently available in the market support transportation in many temperature ranges.
PCMs are materials which change the state of aggregation from solid to liquid or change between two different solid crystallization states over a defined temperature range (phase transition). The process is reversible (reproducible phase transition). PCMs are useful in that they can absorb large amounts of thermal energy per storage volume/mass over small temperature differences between the storage medium and its surroundings.
The materials can also store thermal energy for a period and release the thermal energy gain when needed. A latent heat storage material can fulfill the two tasks depending on their preconditioning or thermal conditioning respectively. Temperature stabilization occurs during the phase transition in both the cases. Liquid PCM emits thermal energy during solidification and prevents a quick temperature drop by doing so. A solid PCM absorbs a large amount of heat during the melting process and prevents overheating. PCM in its liquid phase can prevent hypothermia, whereas a solid PCM can delay overheating.
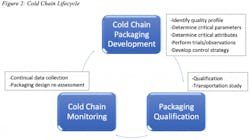
A wide melting range can be used to prevent variables such a high and low temperatures i.e., one chooses to precondition in a way that results in half of the PCM being liquid and half being solid. If two PCMs are used, they are conditioned in a way that the one PCM with a lower melting point is liquid and the other one is solid. This passive way of levelling out the outdoor temperature is supported by sufficient insulation. The latent heat, melting point(s), and the resulting application range are only one part of the system solution for a specific cold chain distribution plan. Each PCMs have their own unique characteristics, as displayed in Figure 1.
Common issues
Product loss during shipment is a problem within the biopharma industry given the economic impact it may have on an organization’s bottom line. The range and the requirements needed to ship biologics is broad. One cause for failure is the segregation or deactivation of nucleating agents when the operating temperature range is exceeded. Potential decrease of stability can happen overtime, such as by the chemical interaction with the PCM environment, the atmosphere, container walls, etc.
Human error events can occur, for example, where supply chain partners forget to place a data logger adjacent to the vaccine to demonstrate robust control during the chain of custody. Freezing temperatures can irreversibly reduce the potency of vaccines required to be stored at 2 to 8 degrees Celsius. Certain freeze-sensitive vaccines contain an aluminum adjuvant that precipitates when exposed to freezing temperature. It must be noted that visible signs of freezing are not necessary to result in decrease of vaccine potency. Although potency of the majority of vaccines can be affected by temperatures that are too warm, the effects are usually gradual, predictable, and smaller in magnitude than the effect from temperatures that are too cold. For example, varicella and influenza vaccines are required to be stored in continuously frozen states and lose potency through the recommended temperature range. Temperature monitoring is the key for proper cold chain management.
QbD approach
A science and data driven approach needs to be considered in developing and qualifying a robust and optimal PCM packing solution. Planning, with stakeholder involvement, where all the product quality related requirements are elicited is a vital part of the process. This process of engagement allows to capture the right product parameters, such as temperature, logistics, and transit routes (domestic/international). Risk management, solution development, configuration management, qualification and transit studies, technical reviews, and traceability are performed as part of the process.
To qualify, PCMs must demonstrate their relevant properties, especially the stored thermal energy, under the application conditions. The solution developed and selected by applying quality by design (QbD) principles needs to take into consideration the supplier reliability, and technical capabilities. The understanding of logistics functions is a critical part of the design development. The distribution environment, understanding the flow of packages as to how they are shaped, handled, and stored are important. Design tests and interpretation of the results needs to be part of the process. Close observation of the package distribution process allows for understanding the variables that impact, the mechanisms of measurement to be employed, determine vibration, drop, compression and the temperature or humidity levels.
The role of responsible packaging design materials is to be consider in current times. Biopharma companies are actively pursuing carbon footprint reduction. The process applied during design captures the critical elements that include functional performance, and environmental performance. Natural resources are considered such that they reduce product damage while reducing environmental impact.
Regulatory and industry standards
Apart from product requirements, there are also regulatory requirements for cold chain solutions:
- 21 CFR 205.50 refers to the minimum requirements for the storage and handling of drugs and details the requirement for temperature recording and record keeping.
- 21 CFR 203.32 discusses the need to maintain the drugs and the conditions to maintain stability, integrity and effectiveness as part of manufacturing specifications.
- 21 CFR 203.36 calls for maintaining forms, reports, and records to meet requirements for record keeping. 21 CFR 211.150 refers to the procedure of how drugs are to be distributed.
- 21 CFR part 11 refers to the regulations and criteria in which the agency considers electronic records and signatures trustworthy.
The international safe transit association (ISTA) develops testing protocols and design standards that define how packages should be to guarantee protection for their content during the transportation cycle. The test is widely used in the biopharma industry to provide confidence in the solutions that are designed for product shipment. There are regional recommendations by different agencies providing guidance on packaging and shipping of vaccines; they need to be referred to while developing a robust cold chain solution.
The U.S. FDA recently released a draft rule on establishing uniform licensing standards for wholesale distributors and third party-logistics providers (3PLs). The purpose of the rule is to provide greater assurance that supply chain participants are sufficiently vetted and qualified to distribute prescription drugs, further strengthening the supply chain. As per the proposed rule, the 3PL must use appropriate manual, electromechanical or electronic temperature and humidity recording equipment or logs to document proper storage. They need to ensure that products are distributed at appropriate temperatures and are under appropriate conditions in accordance with the requirements in the products’ labeling to preserve their identity, strength, quality and purity.
The warehouse/distribution facilities must have equipment that ensures prescription drugs are properly stored, including cold storage, refrigerators, temperature and humidity devices and air handling units. There needs to be monitoring equipment that immediately alert appropriate personnel of any deviations from the required storage conditions. Having robust PCM for cold chain solutions becomes more critical with the planned new requirements for drug distributors and 3PLs.
The use of PCM in cold chain packaging offers customers flexibility, however the selection of the fitting solution warrants attention. The need for testing is predicated on predicting the unknowns while establishing the products shipping configuration; every test performed becomes a steppingstone in the packaging design process. The packaging material tests, and cold chain trial results provide the stake holders with supporting data to make critical cold chain decisions.
A well-documented development, qualification, and monitoring approach for the cold chain packaging and the PCMs utilized will yield the desired results for product owners, distributors, and the end users. A cold chain packaging configuration developed must continually meet its established quality attributes, and operational expectations amid the routine variables in global supply chain logistics.