Bringing down the barriers to process monitoring in biopharma
Biopharma manufacturing depends on living organisms to generate products of interest, and these processes can be extremely complex. Every aspect of a procedure, and its underlying reactions, must be fully understood to ensure optimal process control; the slightest variation in the environment can impact yield and quality.
The knock-on effects — such as failed batches, inefficient use of resources, and end products that don’t meet quality specifications — can be costly and are compounded by a limited availability of data to help drive improvements over time.
Almost any commercial-scale production process — whether it is bulk production of refined oil, gasoline, chemicals and plastics, or the manufacture of end user goods such as pharmaceuticals, foods and beverages — entails numerous checks to ensure the quality of the raw materials, intermediate compounds, and end products. These quality control processes require reliable and accurate PATs that are adaptable to a range of applications, locations and interfaces.
Raman spectroscopy is increasingly being adopted for process monitoring in the biopharma sector, thanks to its combination of speed, accuracy and flexibility. Offering versatile sample analysis that delivers a unique ‘molecular fingerprint’ for each analyte, it enables both qualitative identification of a given substance and quantification of the amount present. Its non-destructive nature makes it ideal for integration directly into production line and process development workflows, enabling continuous process monitoring with in-line or on-line analysis. It is also fast, allowing most substances to be measured in a matter of seconds.
Until recently, Raman spectroscopy demanded complex, bulky and expensive equipment, as well as a specialty personnel to operate and maintain instruments, and there were issues with reliability and cost. The introduction of compact and easy-to-use portable devices with a simplified user interface has been a gamechanger for the industry, providing reliable and affordable solutions that overcome the challenges associated with older equipment for non-expert operators. Manufacturers can now easily integrate Raman spectroscopy into their production processes, helping to improve efficiency and quality without compromising valuable bench space.
In-depth information
Raman spectroscopy provides unique information about a substance — spectra can be obtained from solid, liquid, gas, powder or slurry samples — giving manufacturers the flexibility to monitor their analyte(s) of interest at various points in the production process.
It is particularly beneficial for measurements performed in a bioreactor, as the technique is unaffected by water, allowing monitoring of reactions in aqueous solutions. The direct, linear relationship between the concentration of a given substance and the intensity of the peaks in the spectrum makes it easier to build quantitative models that accurately predict the concentration across the range of detection, even with a relatively small sample set.
This opens applications across the entire biopharma manufacturing process, from verifying the integrity of raw materials to real-time monitoring of bioreactor processes and downstream capture of the product. Crucially, Raman spectroscopy delivers real-time measurements, enabling process adjustments to be made as and when necessary.
Results when and where needed
In-line measurement
In-line measurement involves placing a probe or sampling interface directly into, or in-line with, the process or product flow. This usually means inserting a probe directly into a flow system or bioreactor to continuously monitor the product. The main advantage of Raman spectroscopy is that neither the Raman probe nor sample needs to be removed during the measurement process. This enables parallel measurements at several different locations to determine product consistency throughout the workflow.
On-line measurement
Like in-line analysis, measurements are taken without having to remove samples for testing at a separate location. In this case, a sampling loop is used to separate some of the product from the main process line for Raman analysis. Measurements are performed on just a portion of the product, and the diverted sample can be re-introduced to the process stream or diverted to waste, depending on the application.
At-line and off-line measurement
At-line analysis involves removing samples for testing at or near the process, while off-line measurement involves transferring the sample to a lab setting away from the production site. Today’s compact Raman analyzers — including miniaturized handheld Raman analyzers with quantitative analysis capabilities — are ideal for effective measurements in either of these environments.
Actionable insights
Modern solid-state process Raman systems can readily obtain the near-constant stream of compositional information required to fully understand a bioprocess and run it efficiently. They are stable, reliable and compact — with very few moving parts — and can be placed at any point of measurement to deliver quick, consistent and accurate compositional data, while simultaneously reducing maintenance and operating costs compared to traditional sampling-based measurements.
This data not only provides insights into the underlying reactions, but it can also be processed using data science tools (chemometrics) to develop robust predictive models for key procedures. After validation, these models can be easily deployed to multiple Raman systems to provide actionable information, indicating when feeds or controls should be adjusted to optimize and maintain the bioprocess.
Glucose – a model example
Chemometrics — the extraction of information from chemical systems by data-driven means or modeling techniques — is a major factor in PAT and is fundamental to successful process Raman monitoring and feedback control. An example of this is the glucose feeding cycle, which is essential to enable cell reproduction in bioprocessing. Modeling the glucose content of a system can help to determine exactly when more should be added to maintain a precise rate of cell production.
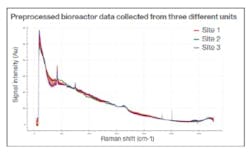
Initially, three Raman data sets were collected from a bioprocess performed at varying global locations, using the same instrument set-up at each site (Figure 1). Despite the small data set, an accurate and precise predictive ‘global’ glucose model was quickly established, using data science tools to clean (pretreat), combine and process the information. The stability, accuracy and consistency of the analyzers ensured that comparable results were delivered regardless of where the devices were deployed, enabling the use of fundamental preprocessing methods that target and amplify the relevant signals within the Raman data.
The predictive model could then be transferred to any other site using the same set-up, and the data outputs used for real-time tracking of the glucose concentration during the bioprocess, as shown in Figure 2, which demonstrates its application to a fourth identical bioprocess and Raman system. The glucose level steadily declines over time until a specified minimum value is reached. At this point, glucose is fed into the bioprocess and the level rises again, ensuring optimal process control.
A flexible solution for all applications
The latest generation of solid-state Raman systems offer enhanced, non-destructive compositional measurements for a variety of sample types, without the need for specialist technicians. Their small size and portability provide flexibility in placement and scalability, making them ideal for real-time monitoring of bioprocesses, opening the door to more applications and facilities. Together with data science tools, Raman spectroscopy can help biopharmaceutical manufacturers to make actionable decisions to better optimize and manage their bioprocesses for maximum efficiency.