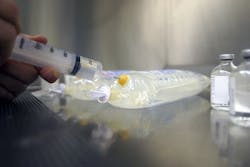
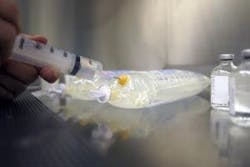
Self-Sealing TPEs for Medical Infusion Therapy Systems
PolyOne GLS Thermoplastic Elastomers now offers Versaflex™ HC for infusion therapy stoppers and septums. In comparison to traditional materials, Versaflex™ HC improves patient safety and reduces manufacturing complexity while streamlining regulatory compliance for faster speed to market.
By replacing traditional stopper and septum materials with insert-molded Versaflex™ HC, PolyOne healthcare customers can achieve:
• Excellent self-sealing: Formulated specifically for resealing applications, Versaflex HC avoids coring contamination that is characteristic of traditional vulcanized rubber, which can leave particles of material behind during needle insertion.
• Resistance to coring: Testing Versaflex HC for resealing and coring performance with needles as large as 16 gauge achieved excellent results including good resealing after multiple penetrations.
• Enhanced purity: Because it does not use sulfur, zinc or other cross-linking agents common in curing vulcanized rubber, Versaflex HC provides superior purity to reduce the risk of leaching and interacting with sensitive pharmaceuticals.
• Reduced manufacturing cost: Versaflex HC processes in a simple, one-step injection molding operation vs. the multiple steps needed for vulcanized rubber. In addition, these materials do not need the washing step required with conventional rubber.
• Streamlined regulatory compliance: This material meets the requirements of the ISO 15759 standard for medical infusion equipment, and can be steam autoclaved using temperatures up to 126°C