Follow-on biologics, aka biosimilars, are close to becoming reality in the U.S. Two separate bills are under consideration by Congress (Box), and passage of either, in some form, would finally give manufacturers guidelines and the green light to make and market generic versions of off-patent biologics.
Yet follow-on biologics (FOB) legislation has been back-burnered as Congress addresses sweeping health care reform. Until the health care debate is settled, the particulars of FOB—what defines a biosimilar, how differing molecules will be treated, what duration of exclusivity originating manufacturers will have, and so on—will have to wait. [Editor's Note: Since publication of this article, health care legislation that is nearing approval favors giving originating manufacturers broad patent protection--click here for one assessment.]
This is a problem, says Matthew Hudes, U.S. Managing Principal for Biotechnology with Deloitte Consulting. If biosimilars legislation is lost amid the “noise” of broader health care debates, it may never get the careful and due consideration it deserves. What’s more, it may end up too closely resembling the Hatch-Waxman Act—the 1984 legislation that laid the groundwork for today’s generic pharmaceutical industry and, critics say, set the tone for the industry’s current malaise. Biopharma could become just another pharma, Hudes says—dominated by major players, saddled with inadequate pipelines and inefficient operations, and lacking of a core spirit of innovation.
Hudes and colleagues, including Jim Hollingshead, Principal for Strategy & Operations, are advocating caution. We spoke to both recently about health care reform and biosimilars legislation.
Implications of Health Care Reform
Health care reform will happen, Hudes says, and have positive results for patients and manufacturers. The main issues of the health care debate are around the wider availability of drugs, and the wider availability of care, he explains. As a result, drug volumes will go up while prices will drop.
“The main response from manufacturers will be to increase their operational efficiency,” he says. “Efficiency hasn’t necessarily been at the top of the list for a lot of companies. Their focus has been more on innovation.”
There are obvious opportunities when it comes to efficiency improvements, Hudes says: better quality control, new supply chain designs, increased automation. Tech transfer and scale-up are also areas where manufacturers can cut costs and speed time to market.
But while some manufacturers are plugging ahead and enacting dramatic operational improvements, the “veil” of health care reform looms, he says. “Things are being put on hold, as manufacturers are concerned about what’s going to happen to the basic economic structure of the industry.”
Some manufacturers may look offshore, to countries where the operating environment is not just cheaper but more economically certain. This is especially true for API bulk manufacturers and fill/finish and packaging operations, Hudes says, but less so for biologics manufacturers who need the capital and scientific knowhow found mainly in the U.S.
Biosimilars: Looking Beyond Exclusivity
The fact that biomolecules are infinitely more complex than their chemical drug counterparts is what has kept the U.S. from enacting biosimilars legislation. At issue is what defines “biosimilarity” and whether follow-on drugs should be considered interchangeable with their reference products. (The EU has been less cautious, approving some dozen follow-on products based upon therapeutic “similarity” rather than equivalence.)
If it is wise, Congress will leave it to FDA to define biosimilars and deal with their varied nuances, Hudes says. The first few biosimilars to get to market in the U.S. will set precedents, he says, but none that would account for all potential varieties of protein- and monoclonal antibody-based products. “You can’t mandate it by fiat,” says Hudes. “FDA will be left with the rule-setting, and they’ll have to do it drug by drug because they are so complex.”
Another concern is that those who run clinical trials and develop biosimilars need to get up to speed on the challenges presented by various molecules, says Jim Hollingshead. “The science just isn’t fully developed yet.”
Much of the discussion around the business of biosimilars has focused on the period of exclusivity given to biologics innovators. Proposed reform legislation addresses the topic, with suggested times ranging from five years to 12 years, or somewhere in between.
Biosimilar Bills Currently Under Consideration
| Bill | H.R. 1427/S. 726 | H.R. 1548 |
| Sponsors | Waxman (House); Schumer (Senate) | Eshoo (House); amended by Hagan/Hatch/Enzi (Senate) |
| Innovator Exclusivity | 5 Years | 12 Years |
| Clinical Studies | • Biosimilarity through minimal clinical studies to “avoid duplicative and unethical clinical testing” • Interchangeability may be mandated; biosimilars labeled and treated as interchangeable |
• Biosimilarity established through clinical, analytical, animal, and immunogenicity testing; all may be waived • Biosimilars not treated as interchangeable |
But longer exclusivity might not necessarily be the windfall to patent holders that it would seem, Hollingshead points out. What additional clinical trials would be required to establish biosimilarity? What testing must be done for immunogenicity? What months and years will be added to development? There are many factors at play.
“You could get a lot of exclusivity but end up with the manufacturer and innovator bearing the full responsibility for anything that goes wrong” once a product gets to market, Hudes says. “The potential for disaster would be great.” One bad biosimilar on the market could set the entire industry back, he adds.
Hatch-Waxman, the Sequel?
Hudes and Hollingshead also have concerns about the look and feel of legislation under consideration. Current FOB proposals are modeled upon the Hatch-Waxman Act, which is a mistake, Hollingshead says, given that the chemical and biological drug industries differ dramatically in both structure and science.
It’s also widely recognized that Hatch-Waxman is responsible for much of what ails Big Pharma today. Some of the unintended consequences of Hatch-Waxman that could also plague the biopharmaceutical industry include:
The “Make Hay” Effect: Given the introduction of generics and a set period of exclusivity, innovating manufacturers are pressed to make as much money as they can, as early as they can. As Hollingshead puts it in a recent paper on the topic (titled “Avoiding No Man's Land: Potential Unintended Consequences of Follow-on Biologics”):
“These lost revenues mean lower return on investment for the innovator, and faced with that prospect . . . they will try to maximize the revenues they can realize ahead of the onset of generic competition. They can do this in two ways: 1) raise prices; and 2) invest more in the marketing of the new drug at launch to drive earlier adoption. This is exactly what happened in the wake of Hatch-Waxman. In this respect, Hatch-Waxman arguably worked counter to its original intent.”
The Blockbuster Effect: Before Hatch-Waxman, manufacturers would develop drugs for smaller markets, knowing they had unlimited time to recoup costs. After Hatch-Waxman, that payback period was cut short, meaning that manufacturers prioritized drugs that had either large patient populations or high value. “There’s no accident that chemical pharmaceutical pipelines dried up” in the years after, Hollingshead says.
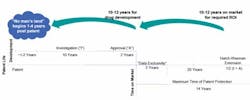
No Man’s Land: As soon as manufacturers commit to a promising drug candidate, the clock begins ticking on its usefulness to the company. If a drug is patented but languishes in development for three or four years, the risk-reward picture muddles and it may never get developed, Hollingshead points out. (See Figure 1.)
These trends point towards the origination of a Big Biopharma in the vein of Big Pharma. The major players in the biosimilar world will be the Pfizers, Roches, and Amgens of the industry who have the in-house expertise and capital to run successful operations, Hudes and Hollingshead say. Larger generics manufacturers will also gravitate towards the market, they say. (Teva has been very aggressive, and recently submitted a Biologics License Application with FDA for a biosimilar version of Amgen’s Neupogen.)
As for the little guy, the startup? There won’t be many opportunities, Hudes and Hollingshead say, because of the resources required to enter the market. “With all the mergers and acquisitions going on, there may be some facilities that open up that would lend themselves to FOB,” Hudes says. But this will be the exception, not rule. And venture capital, the engine of biotech, will shift towards late-stage investments that carry much less risk.
For better or worse, biopharma has greatly matured. The “Make Hay” effect already exists, Hollingshead says. Should a Hatch-Waxman-like bill for FOB be passed, some version of the Blockbuster Effect would kick in. The greatest risk is that the No Man’s Land scenario would apply to an increasing number of early-stage drugs, causing them to be aborted long before their true value (to patients and manufacturers) can be assessed.
Hollingshead writes, “After that point it will never pay back for anyone to develop the compound. Given the overall economics of producing a financially viable drug, we estimate that no man’s land appears very quickly for a new compound—within as little as one year of receiving a patent.”