There are numerous mechanisms for delivering a drug to a patient. Whether tablets or capsules, sublingual solid doses or patches, injectables or suppositories, when it comes to drug delivery solutions, all may be modified for time of API release into the bloodstream. Among these, tablet-based dosage forms have particular complexities which, depending on formulation, can grow exponentially — depending on the modified release (MR) strategy chosen to correctly administer the tablet’s therapeutic dose over time. Process analytical technologies (PAT), fortunately, can help drug makers better understand the choices they make and provide the data they need to better understand MR mechanisms relative to formulation and support a robust and effective QA/QC effort.
There are numerous definitions of MR. Modified release can be defined to mean any “un-natural” mechanisms used to change the bioavailability or rate of uptake of the API by a body. As a baseline, consider a mixture of API, excipients (bulking agents), lubricants and flow agents as the “natural” mode for tablets. That is, no chemical is added to modify the solubility of the API, in any manner.
When a dosage form is modified, it may be to either speed up or slow down the release from the matrix (all the non-active ingredients). The nearest analogy may be a military missile: a standard tablet may be seen as a ballistic artillery shell; the modified dosage form is a guided missile. A number of popular approaches will be covered with suggested techniques that could be used (in a PAT program) to assure proper parameters.
ACCELERATED API BIOAVAILABILITY
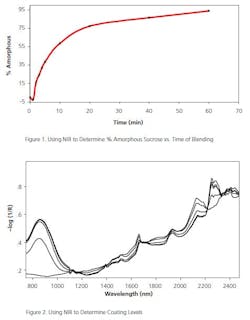
A number of APIs are known to have low or slow solubility in vivo. Physical and chemical modifications to both the API and matrix may be made to speed up the transport to the drug to the blood. One common approach is to keep the API in an amorphous state to aid in its solubility (Figure 1 shows the % crystallinity, followed through a blending process). In this case, a Raman or NIR scan during the granulation and pressing of the tablet can assure that the API was not converted to a more crystalline form.
Another approach is to add a solubilizing material, such as a surfactant, disintegrant or both. Again, a simple NIR approach may be used: a mere reflection spectrum to assure rations or chemical imaging to show distribution of materials.
CONTROLLED RELEASE (DELAYED)
A number of drugs are administered around the clock to maintain effective blood levels. In the “classic” approach, a tablet or capsule may be administered three or four times a day. This assumes patient compliance and, while effective, blood level graphs may resemble a roller coaster ride. To assure more even blood levels, continuous release dosage forms have been developed.
Entero-Coated Tablets: available since the 1960s, these dosage forms depend on an insoluble matrix, usually something like carnauba wax, in which particles of API and soluble excipients, such as starch, are mixed and pressed into a tablet. As the starch dissolves, the solvent reaches the API and slowly dissolves the particles.
It was seen, as far back as the early 1970s, that the rate of release could be predicted through thermal analysis (DSC or DTA). The faster the matrix was cooled or the harder it was pressed into tablets, the higher the energy of the wax polymorph formed. The consequence of the high energy polymorph was greatly slowed dissolution. Today, Raman or NIR can easily monitor (and control) the polymorphic state. Some other MR approaches include:
Film Coating: The most common method has been to modify the type and thickness of the coating. The coating could be designed to dissolve at a set pH (as in the intestine @ 7.5 instead of the gut @ 1.0) or remain intact and allow drug and solvent to pass through in a controlled manner.
Raman or NIR may be used in situ in the coating pan to monitor the correct application of the coating material(s) to the cores in real time. After the coating and/or during development, Terahertz is useful to show, not only the thickness of the coating, but whether or not the coating is properly adhering to the core (Figure 2 shows NIR spectra of a core being coated).
Timed-Release Beads: Or, as commercials put it, “tiny little time pills.” These are usually inert cores, coated with drug, then coated with some coating material(s) (Figure 3, NIR chemical image of time release bead). The thickness of the coating determines the dose level and rate of release. Both NIR and Raman may be used in the spray pans or Wurster coater to control the coating profile.
Reservoir with Polymer Coating: This dosage form is, in essence, a liquid or semi-solid reservoir with a controlling polymer coating to control the release rate (Figure 4). Again, NIR or Raman as controls could allow real-time control.
Drug Dispersed in Polymer (with polymer coating): Figure 5 shows the similarity of this type to the two preceding types. However, mixing the drug with another polymer to give a controlled matrix allows the reproducibility to be somewhat better than the other two forms.
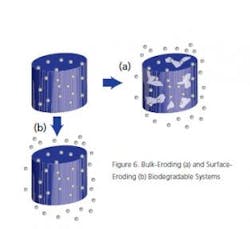
Bulk-Eroding and Surface Eroding Biodegradable Systems: Figure 6 shows the general form of these type of products. They are made from biodegradable (e.g., polylactate) matrices and can be ingested or placed subcutaneously for long-acting drug delivery. Once again, NIR and or Raman may be used for controlling the production of these forms.
Osmotic Pumps/Systems: This is a very interesting approach. The drug is sequestered in a non-soluble “shell,” which has specifically set openings for solvent to enter and begin dissolving the drug. As the solvent is drawn in by osmotic pressure, it eventually forces the drug-containing solution out. As the pressure decreases, more pure solvent is allowed in to dissolve more drug substance and the process continues until all the API is dissolved and forced out. This device is made quite precisely and is controlled by vision systems (pores), NIR or Raman. The device lends itself to the new generation of 3-D printers.
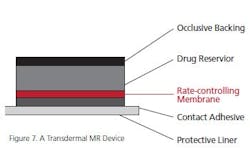
Transdermal Devices: Sometimes referred to as “patches,” transdermal devices can be quite sophisticated. Figure 7 shows how a well-designed device looks in a cut-away picture. Of course, there are as many variables in skin as in gastric systems, so this device is often limited to drugs that could cause gastric distress or are poorly soluble in the gut and intestine.
MULTIVARIATE CORRELATIONS OF IVIVC AND ONLINE MEASUREMENTS
The correlation of in vitro or lab results (usually dissolution) with in vivo or clinical results needs to be demonstrated before an NDA or ANDA is approved by the appropriate Agency (FDA, EMA, etc.). When an appropriate dissolution method is approved for product release, then the company has a tool for both release and stability. However, since the dissolution method often takes between eight and 12 hours, it is hardly an appropriate method for a PAT or QbD program.
To perform in-process measurements (and modifications/corrections), a rapid and, preferably, non-destructive method needs to be found. What is needed is for the PAT group to find a method (i.e., near-infrared, Raman, light-induced fluorescence, terahertz) that will see, qualify and quantify subtle changes in dosage forms as they are produced. These (subtle) changes then need to be correlated with the approved release/stability methodology. To do this, multivariate algorithms, properly handled by a Chemometrican, need to be employed.
What must be done very, very carefully, is not to find a quick and easy correlation and assume it is appropriate. It is likely that this second-tier correlation will take far more samples from far more lots to assure both QA and the FDA (EMA, etc) that what we are reading is true.
These dosage forms are essential for many of the newer drug substances, yet, unless the manufacturing process is made better than the traditional four sigma approach to tablets, the costs will place them beyond most patients. The well-run QbD approach will make these clever, inventive products safe and effective.
Published in the September 2013 edition of Pharmaceutical Manufacturing magazine
Published in the September 2013 edition of Pharmaceutical Manufacturing magazine
About the Author
Emil W. Ciurczak
Contributing Editor
Sign up for our eNewsletters
Get the latest news and updates