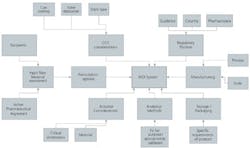
Figure 1: Overview of pMDI input factors.
Pressurized Metered Dose Inhalers (pMDIs) are complex systems and understood to be technically challenging to develop. In order to successfully formulate a pMDI system, there are many factors that need to be assessed and brought together. The formulator must ensure that the finished product is safe and efficacious for the duration of its shelf life.A pMDI system is made up of a number of sub-systems, all of which are required to operate with one another to ensure that the finished pMDI product works correctly. To broadly summarize, these sub-systems include the formulation, container closure system, actuator and secondary packaging (Figure 1). The development of pMDIs requires a total system approach to fully design and optimize the production in order for it to meet its stated design requirements.During critical early-stage development, formulation options are assessed and developed based on a defined, scientific approach. The aim is to repeatedly demonstrate, with meaningful analytical methods, that the formulation is capable of meeting the required product performance criteria. In light of the range of components within a pMDI system that can, and should be optimized and understood (see sidebar, page 14), the following offers an overview of some of the generic activities required to perform early phase formulation and optimization of a robust pharmaceutical pMDI system.
STAGE ONE
Project Preparation and Scoping: During this initial stage, all supplied and public domain information is reviewed. An assessment of project risks is also initiated. This should ensure that all prioritized factors are included in the work plan.
In scoping a project, it is vital that all factors within the plan are considered, even if only a subset may require further investigation. A record of this decision should be kept to help inform any future investigation requirements.
STAGE TWO
Pre-Formulation: During this stage, the API should be characterized. This will involve understanding the key solid-state and physical properties that influence pMDIs. These factors may include polymorphic form, amorphous content, purity and hygroscopicity.
In the first instance, the above factors should be assessed thoroughly at the onset of the project and re-assessed at key times as the project progresses — for example, following particle engineering. Alternatively, specific studies could be performed to investigate the effect of different factors on the above.
Similarly, excipient characterization should be performed on potential excipient options, although it is generally assumed that data may be transferred between projects for common excipients. If a study is required, then tests similar to those performed during Active Pharmaceutical Ingredient (API) characterization should be investigated.
PARTICLE SIZE REDUCTION
Particle size reduction should be undertaken for systems that are likely to necessitate formulation as a suspension product. Note that engineering trials will not be required if a solution formulation route is indicated by an initial theoretical assessment of solubility or solubility study. The aim of a particle engineering study is to identify a suitable process and optimize critical controls for producing size-reduced material. Once a suitable method of particle size reduction has been identified theoretically, a study will typically be performed to establish an operating window and will be based on a Design of Experiment (DoE) type approach. The purpose of this initial study is to generate early data and to allow initial lots to be manufactured.
For the purpose of this article, let us assume that particle engineering is based on jet micronization or spray drying. If adopting a jet micronization process, experimental factors may include product feed rate, ring pressure and Vecturi pressure. If adopting a spray drying process, experimental factors may include product feed rate, aspirator speed and inlet temperature. In each case, parameters will be optimized for Particle Size Distribution (PSD) and yield.
An experimental design, based on a full-factorial design with center points may be utilized. Responses may include:
• PSD by laser diffraction
• Polymorph assessment
• Assessment of change from Input Raw Material (IRM)
The experimental output should identify a suitable process to produce particle size reduced material in the appropriate PSD. It may be desirable to take two or more options forward into further experimental testing; for example, to allow optimization of the Aerodynamic Particle Size Distribution (APSD) to be performed.
SOLUBILITY, GROSS CHEMICAL COMPATIBILITY
Pre-formulation activities will be completed by performing a study to determine the solubility of the API in potential HFA-based systems and to assess the potential for formulation of a suspension or solution-based system. Dependant on the API, it may also be necessary to assess the level of solubility in co-solvent only. Additionally, this study should provide an early indication of solubility-related effects for suspensions, such as Ostwald ripening. This assessment will typically include all propellant options (e.g., Propellant 134a and Propellant 227).
Solubility should be determined at a range of expected operating temperatures of the formulation. For example, cold fill temperature (-60°C), room temperature and 40°C, to understand whether there is potential for manufacturing issues, or else potential for solubility-related issues during storage. In addition, further units may be prepared and stored under stressed conditions (typically 40°C/75% RH) for a minimum of four weeks. The aim of this is to determine any gross drops in content assay, or increase in impurities (if a suitable imps method is available at this initial stage), indicating degradation effects. Ideally, this solubility study should identify a lead propellant on which to base the next stages of the feasibility program.
In summary, the aims of the solubility and initial compatibility study are to identify the solubility of the API in a range of solvent or propellant systems, determine whether a solution or suspension approach is most applicable, and assess the gross formulation compatibility.
Raw Material – Active Pharmaceutical Ingredient (API)
• Solid-state and other key physical properties
• Polymorphic nature of the API and changes of morphology on storage
• Particle size reduction of the API and method of size reduction (if required)
• The physical stability of the API
• Will necessitate assessment when stored as a raw material & when formulated in propellant
• Solid-state changes
• Understanding API particle size changes in the formulation during storage
• Understanding material chemistry
Raw Material – Excipient
• Propellant level / mix (density / hygroscopicity)
• Excipient grade / quality (age, moisture content, solubility)
• Levels of excipients (co-solvents or surfactants) required to produce a robust pMDI formulation
• Impurity / compatibility profiles
• Chemical / physical interactions with API
Container Closure System (CCS) – Can
• Physical performance characteristics of the can, for example, the propensity for deposition of the API onto the can surface
• Understanding potential beneficial effects of can coatings on API degradation and in relation to impurities, extractables and leachables
CCS – Valve
• Materials of construction, for example plastic versus metal valve, stem or elastomer seal type
• Physical valve dimensions
• Processing parameters during manufacture, for example, crimp dimensions
• Confirmation of the need for, and the optimal type of coating
• Interaction of the formulation on the valve
Actuator
• Jet length
• Orifice size
• Droplet size
• Coatings
Secondary Packaging
• The requirement for secondary packaging will vary from product to product, although consideration should be built into all new projects at the start. Areas for understanding and optimization include:
• Prevention of known hygroscopicity issues of a particular formulation type
• Prevention of moisture induced physical and chemical degradation of sensitive APIs
Manufacturing
• The manufacturing process should be optimized for each product to ensure that product quality is maintained. Generally speaking, this should be based on the commercial scale filling lines on which the final product is to be manufactured
STAGE THREE
Formulation (Compatibility System DoE): The majority of formulation activities, including the justification of potential formulations for progressing to later development stages, will be assessed by performing a limited compatibility DoE. This will also include additional systems being tested at centerpoints of the study design with the aim of reducing testing requirements to a manageable level.
With the aim of determining a suitable pMDI system, a limited half to full factorial DoE may be performed focusing mainly on the numeric formulation variables. These variables can include, but are not limited to, co-solvent level, additional excipient level (e.g., surfactant). If required, other factors such as API size reduced material may also be included. At this stage, categorical variables, such as valve type or can coating, may also be included. Testing will include all factors as previously discussed.
As a result of structuring the experiment this way, a test such as APSD, which is labor intensive, could be included on one or both legs of the study, allowing an assessment to be made with respect to the propensity for Ostwald Ripening, without losing the power of the experiment by testing all points. In addition, further system options, which in theory should have a lesser effect on the pharmaceutical performance, would be manufactured to correspond with the DoE centerpoints. Factors could include, for example, elastomer type, valve stem construction and secondary packaging.
This approach would then allow a response surface to be estimated in terms of the main DoE factors, with the possibility to compare the other factors to the DoE centerpoints via use of statistical tools such as an ANOVA or T-test. Further units could be manufactured to allow additional testing to be performed if required.
The advantage of this approach is that it will be possible to perform a robust experiment with limited testing, particularly where tests are labor intensive (e.g., APSD), yet at the same time keep the power of the DoE sections of the study. The study, therefore, aims to allow understanding of potential excipient combinations to progress to later development stages based on the Product Definition. Specific areas of understanding include:
1. Interactions: chemical interactions with API, physical interactions with API and physical interactions with CCS
2. The potential design space window
3. Identification of suitable systems to progress to an informal (short-term) stability
In terms of structure, a compatibility DoE may be based on a full factorial with centerpoints. Experimental factors may include:
• Co-solvent level – x to y% (levels to be defined after solubility testing around solubility co-solvent level)
• Surfactant level a to b% (levels to be defined)
• The effect of different can coating as categorical factor
Additionally, other excipients and propellants may be included, as can additional factors to be investigated at the centerpoint of the DoE only. For example, additional valve options, additional coatings or the effect of foil overwrap and desiccation.
Manufactured units are to be tested initially and following a period of accelerated storage, for example, 40°C/75% RH for six weeks. Testing should include critical performance indicators. These are likely to include APSD of the emitted plume, drug content and impurities. Other responses can be assessed as required. The DoE is designed to provide as output a response surface model of potential system parameters. This will give an early understanding of the system design space with the identification of three to five potential systems to manufacture for a short-term stability assessment of up to six months.
STAGE FOUR
Short Term Stability: Once this stage has been reached, three to five potential systems should have been identified. Batches of approximately 500 units can then be manufactured at a suitable small manufacturing scale, and placed in stability storage chambers for a period of time. The aim of this study is to understand how the three to five systems identified during previous stages perform pharmaceutically on short-term stability and to allow further understanding and definition of the product performance to be assessed.
Stability storage will allow the generation of stability indicating data. The overall aim of this study is to identify two to three suitable systems that may be progressed into further development, including toxicological and human studies. As this study leg is designed to provide stability indicating data with respect to the pharmaceutical performance of a system, the following responses should be investigated:
• APSD
• Content assay
• Impurity level
• Dose content uniformity (DCU)
• Moisture content
• Laser diffraction of emitted plume
• Monitoring of physical properties of API on stability (e.g. polymorphic form)
• Other appropriate testing
Following manufacture and suitable release testing, units will be placed on stability for up to six months. The primary test conditions will be 40°C/75% RH and 25°C/60% RH. Additional units will be placed at appropriate backup conditions. These backup conditions will be based on ICH guidelines and relate to the likely marketing territories. Units may also be placed at a cycling storage condition (i.e. 4°C/40°C at 4 cycles per 24 hours) if deemed appropriate to assess the propensity for solubility related physical incompatibility effects such as Ostwald Ripening.
Consideration should be given to whether a sample or all samples should be foil pouched to further limit moisture ingress on stability. If required, the use of a desiccant within the foil pouch should be included at this point.
The output from this study will provide an understanding of product performance on stability, which will give an initial indication of the potential product shelf life. Assuming the systems perform acceptably during this stage, the aim is to identify two to three lead systems that may be progressed into later development.
Once formulation options have been developed, the product is ready to progress through several more steps. First, the selection of the best product configuration(s) for Phase One and Proof-of-Concept clinical activities, should be based on a thorough understanding of the product performance versus requirements. This involves defining and optimizing the design space, understanding critical parameters and establishing acceptable working ranges. In parallel, methods should be appropriately validated and specifications determined in preparation for a regulatory submission. Secondly, clinical & stability data is generated to further define the product design space in preparation for later development and product registration. And finally, the development of a robust product and manufacturing process, performing Development Pharmaceutics studies and the supply of Ph II / III clinical material.
pMDIs are complex systems and development of a suitable commercialized product remains a technical challenge. However, with a structured approach, the development of a robust, pMDI-based system that meets requirements from a CMC regulatory standpoint is attainable.