Uncertainty and changes to industry trends have created a difficult environment for the current pharmaceutical supply chain. But with challenge comes opportunity.
Pharmaceutical companies are facing challenges that stem from a rise in mergers and acquisitions, a shift to outsourcing of business functions, changes in patient trends and complications with operations.
The surge of mergers and acquisitions and increase in business function outsourcing has forced many underlying business functions to rely on incompatible, legacy data systems. This has led to fragmentation of data across manufacturers, distributors, pharmacies, hospitals/health systems and third-party logistics suppliers.
Additionally, the rise of personalized therapies has been a common trend within the industry. Therapies, such as individualized cell and gene therapies, increase supply chain complexity as future manufacturing is customized and made to order. This shift to customized treatments requires precise product temperature and location tracking to be viable. Within operations, a lack of visibility into manufacturing, aging assets and an underutilized workforce are impacting organizations’ ability to produce and transport drugs efficiently. In fact, product losses due to issues such as temperature variations during transit are costing pharmaceutical companies over $15 billion a year.1
The current challenges within the supply chain have been further magnified through the disruptive force of the COVID-19 pandemic. The outbreak has directly impacted the logistics of supplying drugs to patients, disrupted the ability to plan and schedule production, and further complicated forecasting of demand and capacity.
Organizations can navigate industry disruptions by applying Internet of Things (IoT) technology to improve how drugs are produced and supplied to patients. The adoption of IoT will enable a more nimble supply chain that allows for rapid response during industry turbulence.
Embracing the disruption
We are entering an era where emerging technologies are reaching maturity. IoT has become far more than a futurist prediction — it’s now the backbone of the connected world and a fundamental component of smart manufacturing and the modern supply chain.
IoT will allow data to flow seamlessly from operations and equipment into the hands of workers, management and executive leadership, providing end-to-end visibility and enabling real-time, evidence-based decision-making. The ability to connect, track and analyze processes from sourcing to storage and administration will provide an audit trail of compliance while improving control over inventory and delivery. Analytics will further advance the insights available from IoT data to promote drug product quality, reduce lead times, predict equipment failures, estimate downstream product needs and optimize capacity utilization.Further, IoT will aid in the integration of supply chain data with other functions of the pharmaceutical value chain, including marketing, sales, finance and R&D. Capturing and analyzing supply chain data on shipments, usage, returns, claims and clinical outcomes will provide real-world evidence on drug efficacy. These insights can help pharma deliver the right product to the right patient at the right time.
In this pharma supply chain, changing stakeholder needs and industry trends are met effectively in real time. Leveraging IoT technology improves visibility across the supply chain and enables a more nimble approach to serving patients. The shift to a more intelligent, well-networked supply chain will help organizations cope with disruptions like the COVID-19 pandemic.
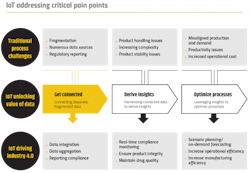
Addressing pain points
IoT has the potential to improve pharmaceutical supply chain visibility and productivity by connecting various data sources, enabling insights and providing optimization opportunities.
A combination of hard and soft sensors will enable organizations to capture data on often-overlooked variables, such as machine utilization, equipment health, process conditions, energy consumption and interplant logistics. Deploying sensors to the highly utilized equipment on a fill-finish line allows manufacturers to monitor equipment uptime in an area where downtime directly impacts the quantity and timeliness of product reaching the patient. Unlocking new data points also allows for the monitoring of critical assets, such as autoclaves and centrifuges, while providing insight into process conditions, such as moisture content in a lyophilizer.
The improved data availability from IoT enables employees to monitor operations away from the production floor and even remotely. When used for reporting, this available production data enables a smart factory that is capable of monitoring operations in real time to improve the physical process control of drug production and material flow.
Outside of interplant operations, IoT enables organizations to integrate the flow of data from suppliers to customers by using cross-platform open source data frameworks. This will allow for individualized therapies, such as cancer treatments, to utilize a fully connected data infrastructure to efficiently transfer information between unaffiliated entities such as hospitals, laboratories and logistics centers. The ability to control the flow of data and bring different sources together also creates value within the organization’s internal operations. Combining different data types unlocks insights not previously available. This new, connected digital ecosystem will enable delivery of treatments to patients in a faster and more secure way.
The use of analytics can further magnify the value unlocked by applying IoT to the pharma supply chain. Analytics, such as machine learning and artificial intelligence (AI), can predict equipment failures, forecast batch yields, sequence equipment efficiently and estimate downstream product needs. In fact, it is estimated that more than 50 percent of pharmaceutical and biotech manufacturers will employ prescriptive analytics and AI by 2021.2 The availability of this forward-focused information enables smart manufacturing practices that improve overall equipment effectiveness (OEE), increase revenue and boost workforce productivity.
Augmented reality/virtual reality (AR/VR) further increase the value a supply chain will see from a digital IoT transformation. These technologies allow for improved data visibility in a hands-free environment, more effective trainings for operation employees and the ability to support and problem-solve operation critical employees remotely.
Embarking on the IoT journey will act as a supply chain accelerator by bringing the plan, source, make and deliver functions in closer alignment with the strategic imperatives of the organization as it becomes nimbler and more responsive.
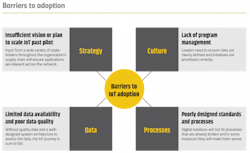
There are no shortcuts
Thirty percent of IoT projects fail in the proof-of-concept stage. This isn’t a reflection of the technology; there are many successful deployments.
There many successful deployments. However, too many businesses try to begin the journey despite badly implemented processes, unclear roles and responsibilities, poorly designed organizational setup and other issues. These problems need to be resolved before the push to digital can begin.Embarking on an IoT journey is a significant, company-wide responsibility demanding the dedication of substantial resources and investment across multiple departments. Because the groups of stakeholders come from fundamentally different worlds, it is critical to establish a common ground from the beginning. Creating an IoT Center of Excellence (CoE) will align strategy with execution by ensuring shop floor stakeholders are able to provide thought leadership and direction to the team executing on the IoT journey. Enabling team members to work in parallel with end users fosters an environment of continuous learning and communication.
Adoption is equally as important as execution. The organization is only able to extract the full value from IoT technology if it is effectively used throughout the supply chain. To adopt the technology in the most impactful way it is important to consider necessary changes to SOPs and ways of working. This concentration on adoption ensures the program maintains a forward focus on scalability and the realization of value.
The IoT journey
Frame the CoE scope and scale: Pharma organizations should first build a foundation by understanding the current context and future direction of the supply chain in which the CoE will operate. A detailed diagnostic analysis is conducted to understand digital readiness and identify where digital capabilities align with challenges facing the organization. This first step on the digital journey sets the expectation within the organization on what level of investment and type of infrastructure improvements will be required.
Establish the CoE operating structure: Now that expectations and direction have been set, the organization needs to design its operations to be able to execute. Within the CoE, reporting lines and team structures should be redrawn with digital in mind to create cross-functional teams that eliminate siloed work. Agile training will enable these cross-functional teams to efficiently deliver capabilities that empower employees throughout the supply chain. To enable CoE members, agile SOPs that align with the digital mindset and ensure transparency should be implemented. Measuring the success of the CoE is also important. Leadership needs to define and monitor key performance indicators (KPIs) to understand the team’s achievements and identify improvement areas.
With the agile ways of working established, pharma organizations must prepare technological infrastructure by identifying a development platform, defining system architecture standards and building analytics capabilities. Cross-platform data frameworks should be implemented to enable the flow of data between different source systems and entities. To ensure the right decisions are made it is important to align the direction of the organization with its current digital maturity and technology needs. An assessment can help steerdecisions that directly impact the development feasibility of IoT applications.Identify and launch IoT pilots: With a solid operating structure established, the next step is to define strategic priorities for IoT initiatives. A top-down/bottom-up approach allows the steering committee and shop floor end users to set the direction, reducing the likelihood that a one-off solution is developed. Many organizations that are working to improve smart manufacturing capabilities will conduct an assessment of current operations and prioritize quality control, energy monitoring, predictive maintenance and materials management for IoT application development. When deciding on pilots it is important to consider criteria like ease of delivery, network applicability and business value.
After opportunities are identified and agreed upon, it is time to execute on developing the IoT applications as pilots or proofs-of-concept. The pilots are important for gathering stakeholder feedback and should be measured to demonstrate business value before the solution is scaled throughout the organization.
Instill a digital mindset and conduct change management: Running a successful IoT transformation program is not just about tools and technology; it’s also about the people. A digital mindset is crucial for building a performance culture that delivers and adopts digital solutions effectively. This digital mindset needs to be adopted at all layers of the organization to create ownership and ensure effective communication. For shop floor employees, digital working methods need to be implemented that maximize the effectiveness of IoT technologies while enabling workers to focus on higher value add activities. Conducting a current-state shop floor team capability assessment will allow the organization to understand where training is required. A training road map or plan should be devised that includes interactive exercises on how to use the new technology. Outside of IoT adoption, it is important to upskill employees to perform tasks outside of their current skill sets.
Scale and sustain: Without upkeep, a digital transformation is sure to fail. Deploying IoT solutions on a large scale will impact all functions, including finance, human resources, sales and R&D. It is important to work with these areas of the business to ensure all functions are pursuing the digital journey with a similar mindset and integration points are considered.
Scaling best practices and sustaining a digital transformation requires resources to maintain system architecture, monitor application usability, document business impact and follow application life cycle management processes. Scaling and rapidly deploying IoT capabilities across manufacturing and supply chain operations will directly impact the return on investment (ROI) for the CoE and IoT program.
Taking the first step toward the giant leap
The time is now for companies to make a move or risk spending years struggling to catch up later. To best understand the opportunities inherent in IoT adoption, companies should begin to assess which areas of their supply chain are best suited for the technology. A clear plan that ties into the company’s overall strategy and creates buy-in from stakeholders can provide that first step.
Success will require a lot of experimentation. Setting reasonable expectations at the start, promoting frequent conversations between developers and users and providing competent training will facilitate the trust needed to achieve the vision of an intelligent, networked future supply chain. If all done correctly, IoT can help the supply chain adjust to shifts in industry trends and navigate unforeseen disruptive forces like COVID-19.
References
1. FedEx, “Harnessing Big Data to Transform Pharmaceutical Logistics.” Dec 2018.
2. IDC FutureScape: Worldwide Health Industry 2020 Prediction. 2019.