ATMP facility design basics for international compliance
Being CGMP compliant is particularly important for advanced therapy medicinal products (ATMP) manufacturing, especially autologous cell therapy products, due to the critical time constraints of production and ‘one-shot’ personalized nature of many therapies.
In late May, the FDA updated its Facts About the Current Good Manufacturing Practices (CGMP) overview document. The document reminds readers that the “FDA has announced a number of regulatory actions taken against drug manufacturers based on the lack of CGMP.”
The FDA also notes that “CGMP regulations for drugs contain the minimum requirements.” Many pharma manufacturers, the update acknowledges, “are already implementing comprehensive, modern quality systems and risk management approaches that exceed these minimum standards.”
It’s important to remember, however, that CGMP guidelines are not limited to those developed by the FDA. Consequently, CGMP guidance from other locales including the European Union should be considered to ensure compliance with a broader audience.
A good understanding of the similarities and differences between U.S. and EU practices is essential in the development of ATMP facilities. Among other reasons, a company is limiting itself from selling products internationally, if those products come from facilities that only follow U.S. CGMP regulations.
Let’s look more closely at some of the differences in CGMP requirements domestically and internationally.
Published guidance, here and abroad
For the sake of brevity, we will focus on our comparisons here between the requirements set out by the FDA for products being sold in the U.S., and the European Medicines Agency (EMA) for products being sold in the EU. For the most part, the EMA’s guidelines are very similar to those of the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S), which harmonizes and establishes GMP standards for the rest of the world.
It makes sense that there would be two completely different sets of procedures for compliance domestically and abroad. In Europe, the EMA specifies requirements for ATMPs: EudraLex Volume 4, Part IV – GMP requirements for Advanced Therapy Medicinal Products
The U.S. issues guidelines through the Code of Federal Regulations, specifically, 21 CFR Parts 210/211, 1271, and 600. Additionally, the FDA has separate guidance for industry specific to human cellular products: Guidance for Industry: Current Good Tissue Practice (CGTP) and Additional Requirements for Manufacturers of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)
Both the EudraLex guidance on the Manufacture of Sterile Medicinal Products and the FDA’s guidance on Sterile Drug Products Produced by Aseptic Processing are often followed together, due to the nature of autologous cell therapy manufacturing. These products cannot be terminally sterilized, which necessitates aseptic processing throughout the manufacturing process.
Cleanroom classification differences
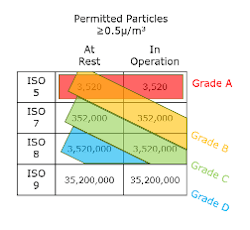
The FDA references ISO 14644-1:2015, the ISO System for cleanroom classification. This system indicates in-operation requirements only. The EU, however, uses the Grade system, which for a given Grade, defines requirements both in operation and at rest. EU classifies and monitors airborne particle sizes at two thresholds: ≥0.5 um and ≥5.0 um, whereas FDA only has a single threshold of ≥0.5 um. This can often cause confusion when defining cleanroom requirements. The below table reflects the correlation between ISO and Grade both in operation and at rest.
As noted in the table, the EU’s Grade D classification has no equivalent designation by the FDA’s ISO system. Consequently, isolator background environments have different restrictions dependent on which guidelines are being followed.
In the EU, prescriptive guidance on ATMP manufacturing is very thoroughly detailed. It specifically notes that open manipulations must occur in a Grade A environment (for example, a biosafety cabinet), with a Grade B background. It also allows for the use of a Grade D background if open operations are contained within a fully closed environment such as a Grade A isolator.
By comparison, the FDA requires an ISO 7 (equivalent to a Grade C) background. As indicated in “Guidance for Industry: Sterile Drug products Produced by Aseptic Processing,” the environment of the room surrounding the ISO 5 environment should be ISO 7 or better.
Accelerated designations
Many ATMPs may be classified with special designations that may offer benefits from regulatory agencies. Benefits can include priority approval, tax credits and extended exclusivity for marketed products. Each program has distinct benefits and sponsors can apply for more than one approval.
What follows is a brief overview of just some of the designations appropriate to ATMP production.
Both the EMA and FDA have an orphan drug designation, but with specific differences in how these rare diseases are quantified. In the U.S., for example, an orphan indication is defined as a prevalence of fewer than 200,000 persons. By contrast, the EU classifies orphan diseases as affecting fewer than five out of every 10,000 persons.
Two other common accelerated designations in drug development include Fast Track and Breakthrough (or 'PRIME' in the EU).
The Fast Track designation is intended to expedite INDs for serious conditions that address an unmet medical need. This designation can be based on non-clinical or clinical data and may come with authority to expedite product review. This designation can also enable priority review. The review time is shorter in the U.S. — approximately 6 months for FDA review, compared with an average of 10 months.
The Breakthrough designation in the U.S. expedites the development and review of new medicines for serious or life-threatening conditions. This designation stresses improvements measured by the impact on clinical endpoint data. Benefits of this designation include FDA guidance, rolling reviews, and expedited approval processes.
The equivalent of Breakthrough in the EU is what’s known as “PRIME” (PRIority MEdicines). The primary target for this designation is small companies or academic spin-offs, and stresses the importance of therapeutic innovation.
An additional designation in the U.S., as denoted by the FDA is Regenerative Medicine Advanced Therapy, or RMAT. This designation is exclusive to cell and tissue-based therapies intended to treat or cure life threatening conditions. The RMAT designation includes all benefits of Breakthrough and Fast Track. It is intended to address the more complex nature of designing clinical trials for complex biological products, such as cell and gene therapies. To date, some 62 requests for RMAT designations have been granted, with more than half for autologous or allogeneic cell therapy products.
It is advisable for U.S.-based companies to design their ATMP facilities to comply with both FDA and EMA requirements. Companies that don’t have international approvals for their ATMP products now must still design to international standards. If not, they face the unwelcome prospect of retrofitting their facilities to ensure compliance with these global guidelines.