For decades, scientists have viewed mRNA as a promising vaccine and drug candidate. It is easy to synthesize and customize, making it an adaptable platform with a wide range of potential uses. The SARS-CoV-2 pandemic then demonstrated its utility on a global scale, as pharma companies lined up to produce this lifesaving ‘quick fix’ en masse.
However, this meteoric rise has proven to be a double-edged sword. While the pandemic helped alleviate certain unknowns in the use of mRNA as a drug — like proving the safety of lipid nanoparticles (LNPs) — it created new questions which may have long-lasting effects on the field and what indications are tackled in a post-pandemic world.
The role of lipid nanoparticles
Despite the benefits of mRNA, its candidacy as a potential solution to COVID-19 hinged on the pharma industry’s ability to successfully integrate it into manufacturing processes to enable mass vaccination. Thus, the need for durability took center stage as pharma quickly adopted LNPs to ensure mRNA’s stability both in shipping and storage, and when it is in the body.
For mRNA to function as a safe and effective therapy, key biological and manufacturing issues had to be overcome. These included drug targeting, stability, unwanted immunogenicity, and relative translation efficiencies. But potentially more impactful was the choice of LNPs and the associated manufacturing apparatus that was scaled up to produce unprecedented quantities.
The use of LNPs as a delivery mechanism for mRNA vaccines underscores the inability of mRNA to stand alone as a therapeutic modality. For mRNA to have any therapeutic effect, the body needs to translate the encoded message into a protein with the desired biological activity. Inherent in this challenge is that mRNA itself is notoriously unstable, with a very short half-life. As the mRNA degrades, so does the template that codes for the protein conferring therapeutic value. Because mRNA does not incorporate into the genome the way a lentivirus-based gene therapy would, mRNA is a tough sell as a medicine simply from a bioavailability and durability standpoint.
These challenges have driven different groups around the world to research and explore how to make mRNA more stable and viable for longer periods of time. One option is to design and synthesize circular mRNA strands, removing an available ‘end’ for an enzyme to digest and extending the window for protein expression. This is one of several avenues that could be explored.
However, in the urgent rush of the pandemic, LNPs became the go-to method, to the exclusion of all others.
Going beyond LNPs
In practice, an LNP effectively consists of a few types of lipids for structural properties (like cholesterol) and a special, ionizable lipid, which allows the LNP to achieve intracellular delivery. The LNP formulation also acts as an adjuvant to activate the immune system, which is essential for vaccine development but problematic in other applications (although recent research suggests this can be customized to be tolerogenic). This general mixture dates back at least a decade, and the specific formulation used for the SARS-CoV-2 vaccines was not novel, having previously been tested in humans for other indications.
While LNPs were well suited for rapid use in SARS-CoV-2 vaccines, they are a suboptimal tool for unleashing the full promise of therapeutic mRNA. Their overall delivery efficiency is poor, and they lack real organ or tissue targetability.
For the field to truly commandeer our own bodies as ‘drug factories,’ as Moderna’s CEO Stephane Bancel has touted, the world will need something better than an LNP. Yet, the industry now has an almost unlimited capacity to produce them, and as a result, biopharma has rallied around their use, indirectly shaping their pipelines in profound ways.
Short-term fixes for long-term issues
At the start of SARS-CoV-2 vaccine development, there were considerable supply chain challenges limiting the manufacturing of LNP-mRNA formulations. Variable costs and unpredictable supplies of raw materials complicated manufacturing plans. Upstream processing costs were influenced by materials such as enzymes for in vitro transcription and mRNA capping, while downstream processing costs were swayed by the method of purification and overall process yields.
The pharma industry was (and still is) experiencing a labor shortage, driven by a lack of the experienced operators needed for specialized manufacturing processes. With limited human resources, pharma companies faced challenges in bringing production up to required levels and expanding their manufacturing capacity to meet unprecedented global demand.
To alleviate these pressures, LNP-mRNA innovators entered into various strategic agreements to ensure both critical material supply and manufacturing capacity. Examples include Moderna partnering with CordenPharma for its lipid excipient supply, and with Lonza for additional drug product manufacturing capacity. For its part, Pfizer partnered with Acuitasfor LNP delivery technology. Raw material suppliers also rose to the occasion. Avanti Polar Lipid was acquired by Croda mid-pandemic, while MilliporeSigma increased its lipid production capacity by an astounding 50 times.
Longer-term, as the mRNA industry continues to evolve, there is a concern that the collective focus on applications requiring relatively small quantities of mRNA may generate manufacturing challenges. What will happen to all of the production and formulation capacity that came online at the height of the pandemic? If these facilities sit idle, there could be a spike in production costs compared to pandemic levels. This would inevitably impact earlier-stage companies in this space as they vie for funding and growth.
Bolstered pipelines or more of the same?
Unfortunately, the newfound availability of lipid inputs may have created a barrier to further innovation in delivery sciences.
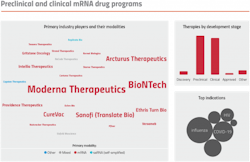
The industry was quick to coalesce around the use of LNPs at a time when interest and investment dollars were pouring into the field of mRNA-based therapeutics. This laser focus limited the pursuit of alternative solutions. The mRNA drug pipeline is now dominated by candidates targeting applications amenable to delivery using LNPs. This includes vaccines and other low-dose indications, or drugs for liver-associated diseases. According to DeciBio’s therapy tracking platform, TheraTrack, over one-third of public preclinical and clinical mRNA drug programs are vaccines. Of the remaining programs, another third target hepatic diseases or rare indications.
Even in good economic times, targeting indications with a lot of competition and a limited market opportunity is ill-advised. But as we look ahead to the second half of 2022, the general market forecast looks far gloomier. This makes pipeline prioritization an even more important part of any biopharma strategy. Companies need to review the landscape and carefully determine what their first and potentially second program will be and how they will be differentiated. Thus, when the market slows down, they’re only focusing on programs that are viable, to begin with — there’s no need for massive and immediate cuts to a number of other programs.
On a more positive note, this type of lean and efficient pipeline can make for a compelling acquisition target for larger pharma.
Future implications
The pandemic established the safety of LNPs and mRNA-based therapeutics — several billion times over. While valuable, this rapid progress may have also unintentionally stifled innovation and exploration of better delivery solutions and technologies. These advances are needed for mRNA to live up to its promise. In the short term, biopharma companies should carefully consider their pipeline and targets to limit later-to-market drugs in indications that will not support them. Moderna may have the resources required to take many shots on goal, targeting many different diseases, but most therapeutic mRNA companies do not. For the health of the field, biopharma companies need to divide and conquer.
While LNPs are ideally suited for various vaccination strategies, therapeutic developers focusing on other mRNA therapeutics (e.g., protein replacement) must carefully consider their targeting strategies. For instance, novel LNP formulations may present additional targeting and delivery options (e.g., switching to pH-dependent ionizable lipids over cationic lipids). Manufacturers of raw inputs need to consider how to scale back their production to suit both extremes of demand, and formulators should be conscious not to take capacity offline at a rate that would cause a shock in production costs and stifle this emerging therapeutic modality.
For these reasons, successful go-to-market strategies will need to balance a therapeutic landscape driven by LNP formulation R&D, nurturing a strong material supply chain and navigating shifting manufacturing capacity
Contributing authors from DeciBio: Shaurya Gilani, Senior Analyst; Michelle Atallah, Life Science Expert; Joe Daccache, Life Science Expert; Rebecca Burnham, Associate Product Manager; Alex Amram, Analyst