Over the past few years, significant increases in protein expression levels have resulted in shortages in the separation, filtration and chromatography capacity required to purify biopharmaceuticals downstream.
Avoiding downstream bottlenecks is a key challenge to ensuring adequate biopharmaceutical supply, and manufacturers and technology vendors alike are evaluating new options for improving downstream efficiency.
This article summarizes results from BioPlan’s Ninth Annual Report and Survey of Biopharmaceutical Manufacturers, from the standpoint of downstream capacity. This year’s results suggest that downstream shortages have eased somewhat, moving from being an “acute” to a “chronic” problem. Nevertheless, they continue to have an impact on budgets and overall manufacturing.
Examining results from the past few years, we see that, this year, more respondents (71.9% vs. 68.5% last year) feel that their facilities are experiencing at least some degree of capacity bottleneck as a result of downstream processes. That is the largest percentage since 2009 (74.5%), and is partly the result of continuing upstream improvements, without concurrent downstream fixes.
However, by breaking down these responses, we can also identify trends in the severity of the problem. Our study shows that 8.5% of the 302 global biopharmaceutical manufacturers surveyed are experiencing a “serious” bottleneck due to downstream processing. The extent to which downstream processing has contributed to serious bottlenecks has varied over the years — in 2008, a low 4.6% of respondents said this was the case; last year, 11.8% said the same.
Although relatively few in the industry are experiencing “serious” bottlenecks, the industry continues to see “some” (37.8%) or “minor” problems (25.6%) due to downstream processing. We note a definite upward trend in the percentage of respondents who see minor problems (up from 19.1% in 2008 to 25.6% this year). At the same time, there appear to be minor downward trends in the percentage who don’t expect a bottleneck (from 21.7% in 2008 to 17.1% this year). In addition, the percentage who don’t have a bottleneck but expect one in the next year declined from 14.5% in 2008 to 11% this year). Taken together, these results suggest that the respondents who previously were not experiencing a bottleneck or not expecting one are now seeing minor problems from downstream processing. There are both good and bad connotations to this: Obviously, the trend toward more widespread capacity constraints is disturbing; but it is encouraging that facilities newly experiencing problems are saying they are minor, rather than more severe issues.
Our survey suggests significant differences in impact when sorting by business model and geography. For instance, Western Europeans appear to be somewhat less concerned by downstream processing than American respondents. This year, slightly more than 4 in 10 (40.7%) European respondents said they were not experiencing any bottlenecks due to downstream processing, more than double the proportion of Americans (17.1%). Over the past 5 years, the proportion of Western Europeans not expecting any bottlenecks has grown from 11.1% in 2008 to 29.7% this year, while the percentage experiencing “serious” capacity problems has shown a correspondingly large decline. American respondents have indicated the reverse pattern, suggesting that while problems remain on both sides of the Atlantic, Americans’ struggles are growing at the same time as Europeans are beginning to resolve their issues.
There are also differences between biotherapeutic developers and contract manufacturing organizations (CMOs). Over the years, the percentage of biodevelopers experiencing “some” or “serious” bottlenecks as a result of downstream processes has gradually risen, from 42.5% in 2008 to 50% this year. Concurrently, the proportion of CMOs indicating these levels of constraint has seen a dramatic drop, down from 68.8% in 2008 to just 25% this year (with none of those 25% being serious problems). This is good news for the industry: If CMOs — who have strong interests in improving throughput — are reducing the problems, it is likely these performance improvements will impact biodevelopers in the near term as well. That is, if CMOs’ experiences are a leading indicator of bioprocessing efficiency, the overall trend towards increased problems may soon level off.
What is the Industry Doing About It?
New downstream technologies are emerging as the industry focuses on alternatives. However, in the near term, many of the incremental improvements, such as streamlining processes and eliminating purification steps, have reduced the urgency for innovative technologies in this area. Prior year studies showed that respondents believed they were faced with a relative lack of options beyond membrane technologies. This is also changing, as new technologies are being introduced. The industry is likewise shifting its assessment of different solutions.
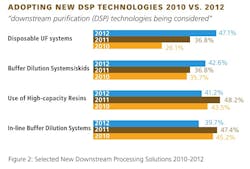
This year, we identified 19 different new downstream purification technologies being considered. The most commonly mentioned bottleneck and DSP problem being disposable UF systems (47.1%); buffer dilution systems/skids (42.6%); and use of high capacity resins (41.2%). Closely following were in-line buffer dilution systems, single use disposable TFF membranes, and single-use filters. On the other end of the spectrum, few are looking at solutions such as alternatives to chromatography (19.1%), precipitation (13.2%), moving beds (8.8%), and small substrates (2.9%).
Interest in some technologies has grown steadily, while it has dropped off for others. The following are some technologies in which the industry has showed significant interest over the past few years: disposable UF systems, buffer dilution systems/skids, single-use disposable TFF membranes, single-use prepacked columns, membrane technology and development of MAb Fragments.
Here again we find significant differences in approach when segregating by business model and geography. U.S. respondents are more likely to be considering technologies such as disposable UF systems, use of high capacity resins, and in-line buffer dilution systems, while European respondents are more favorable towards continuous purification systems, single-use prepacked columns, and membrane technology.
CMOs show a much greater interest than biodevelopers in use of high capacity resins, single use disposable TFF membranes, and continuous purification systems, while biodevelopers are more focused on buffer dilution systems/skids, single use filters, membrane technology, and precipitation, among others.
But What Is The Industry Actually Doing?
While it’s worthwhile to explore what new solutions are generally piquing the interest of global biomanufacturers, it’s also very relevant to examine the specific areas respondents’ facilities have actually implemented in an effort to improve their downstream purification operations.
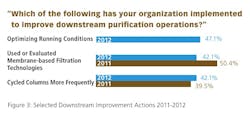
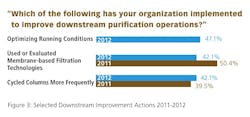
We evaluated 18 factors being undertaken today. The most common approach that respondents are taking to reduce DSP problems is to simply “optimize running conditions,” which was cited by 43.4% of respondents (Figure 2). A similar proportion have used or evaluated membrane-based filtration technologies, and cycled columns more frequently. Other actions included “developed downstream processes with fewer steps” and “reduced the number of process steps.” Only about one in 10 have switched to alternatives to Protein A, an area in which there appears to be more interest than actual activity.
Generally, the areas that we also identified in last year’s study have seen a decrease in the percentage of respondents indicating implementation this year. Those include “use or evaluation of membrane-based filtration technologies,” “development of downstream processes with fewer steps,” “investigation of single-use disposable downstream technologies,” and “use or evaluation of ion exchange technologies.” The only area to see marginally higher take-up this year was “more frequent cycling of columns.”
There are a couple of different ways to interpret this trend. It could suggest that the industry is gradually moving towards a better balance in its downstream capacity, and that the recent bottlenecks are beginning to abate. This reading would follow the signs we found earlier, that while more facilities are experiencing bottlenecks, the problems are relatively minor in scope. This in turn could be the result of generally improved productivity in downstream purification operations, the implementation of better process monitoring, and/or improved technology adoptions.
Alternatively, the trend might indicate that respondents have already implemented the common operational improvements, so this year the industry is looking at other options. Indeed, the fact that we identified 19 DSP improvements suggests the industry is still seeking a cure.
Both CMOs and Western European respondents report a significant decrease in DSP bottlenecks this year.
And we found that in general, these groups differed in their approaches. Both CMOs and Western Europeans indicated use or evaluation of membrane-based filtration technologies to be their most common implementation area: 63.6% of CMOs reported having done so (compared to 38.5% of biodevelopers), and 52% of Western European respondents also reported doing so, compared to 35.9% of U.S. respondents.
This is an interesting development and it will be useful to monitor over time.
On the whole, results of this year’s study indicate a less dramatic impact from downstream processing on overall productivity and capacity. Biomanufacturers’ incremental fixes have moved the DSP pain from ‘acute’ to ‘chronic’ as problems are addressed. Perhaps as a result of these gradual fixes, the interest manufacturers are showing in more novel DSP approaches is declining, or being focused on fewer, more promising alternatives.
References:
1. 9th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production: A Survey of Biotherapeutic Developers and Contract Manufacturing Organizations, April 2012, BioPlan Associates, Inc., Rockville, MD, www.bioplanassociates.com
About the Author:
Eric S. Langer is president and managing partner at BioPlan Associates, Inc., a biotechnology and life sciences marketing research and publishing firm established in Rockville, MD in 1989. He is editor of numerous studies, including “Biopharmaceutical Technology in China,” “Advances in Large-scale Biopharmaceutical Manufacturing”, and many other industry reports. Contact information: [email protected]; 301-921-5979; www.bioplanassociates.com.
Survey Methodology: The 2012 Ninth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production by BioPlan Associates yields a composite view and trend analysis from 302 individuals at biopharmaceutical manufacturers and CMOs in 30 countries. It also included 185 direct suppliers of materials, services and equipment to this industry. This year’s survey covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment.