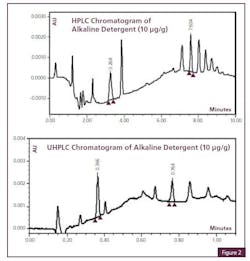
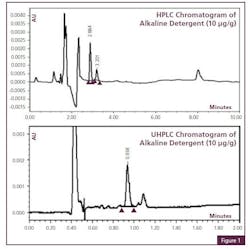
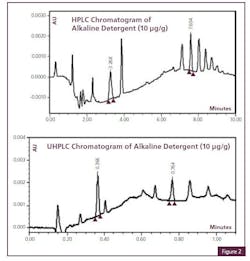
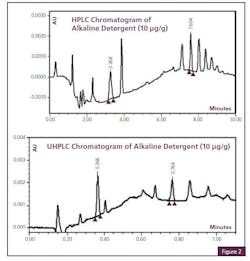
Cleaning Agent Residue Detection with UHPLC
References
[1] APIC Guidance on aspects of cleaning validation in API plants, 2000.
[2] Swartz, M.E., UPLC: An Introduction and Review; Journal of Liquid Chromatography & Related Technologies, 28 (2005) 1253-1263.
[3] Guide to Inspections Validation of Cleaning Processes; FDA, ORA, 1993 pp 1 – 6.
[4] Beilin, E., et al. Quantitation of acetol in common pharmaceutical excipients using LC-MS. Journal of Pharmaceutical and Biomedical Analysis, 46 (2008) 316-321.
[5] Nozal, M.J., et al. Development and validation of an LC assay for sumatriptan succinate residues on surfaces in the manufacture of pharmaceuticals. Journal of Pharmaceutical and Biomedical Analysis, 30 (2002) 285-291.
[6] Queralt, M., et al. Total organic carbon (VCSN and VWP) and HPLC analysis for cleaning validation in a pharmaceutical pilot plant. PDA Journal of Pharmaceutical Science and Technology, 63 (2009) 42-57.
[7] Arayne, M. S., et al. Cleaning validation of ofloxacin on pharmaceutical manufacturing equipment and validation of desired HPLC method. PDA Journal of Pharmaceutical Science and Technology, 62 (2008) 353-361.
[8] Dong, M.W., et al. A Generic HPLC/UV platform method for cleaning validation. American Pharmaceutical Review, Volume 15, Number 6 (2012) 10-17.
[9] Simmonds, E.L., et al. Evaluation of LC-MS for the analysis of cleaning verification samples. Journal of Pharmaceutical and Biomedical Analysis, 40 (2006) 631-638.
[10] Kaiser, H., et al. Measurement of organic and inorganic residues recovered from surfaces. Journal of Validation Technology (1999) Volume 6, Number 1.
[11] Akl, M. A., Validation of an HPLC-UV method for the determination of cefriaxone sodium residues on stainless steel surface of pharmaceutical manufacturing equipments. Journal of Pharmaceutical and Biomedical Analysis, 55 (2011) 247-252.
[12] Zayas, J., et al. Cleaning validation 1: Development and validation of a chromatographic method for the detection of traces LpHse detergent. Journal of Pharmaceutical and Biomedical Analysis, 41 (2006) 589-593.
[13] FDA, Guidance for Industry, “PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance” (Pharmaceutical cGMPs, September 2004).
[14] Rathmore, S.A., Case Study and Application of Process Analytical Technology (PAT) Toward Bioprocessing: II. Use of Ultra-performance liquid chromatography (UPLC) for Making Real-Time Pooling Decisions for Process Chromatogrphy., Biotechnology and Bioengineering, vol. 101, No. 6, December 15, 2008.
[15] Sharnez, R., et al. “Cleaning validation challenges for bioprocesses: strategies for eliminating tenacious residues”, Presentation at 2012 Parenteral Drug Association (PDA) Annual Meeting.
[16] Rathore, A.S., et al. “Case study and application of process analytical technology (PAT) towards bioprocessing: use of on-line high-performance liquid chromatography (HPLC) for making real-time pooling decisions for process chromatography.” Biotechnology and Bioengineering, (2008) Jun 1; 100(2):306-16.