Over the past few years, Tunnell Consulting has studied the tactics and results of a number of organizations as they strove to apply Lean and Operational Excellence (OpEx ) principles to improve the efficiency of their quality control (QC) labs. Some were clients and others were cases gleaned from publications All had a great deal in common in terms of the issues and performance challenges that drove them to seek improvement. While most achieved an acceptable return on investment for their efforts, some achieved extraordinary results. In the case study that follows, we’ll describe how one high-performance organization integrated Lean Lab into a comprehensive transformation of its manufacturing supply chain and achieved outstanding results, which synergistically enabled improvements upstream and downstream in the manufacturing process.
Our case study involves a pharmaceutical company with a large number and variety of products — solids, liquids, aerosols, capsules and tablets. This created significant complexity in the scheduling, queuing, coordinating and conducting of tests. Prior to the Lean improvement project, lab throughput time — the elapsed time between the arrival of a test sample in the lab and the completion of its testing — stood at 15 days. The organization established a goal of 30% reduction in throughput time within three months, and greater reductions over the long term.
In order to realize the full potential of cost savings, it was imperative to increase Right-First-Time (RFT) results.
There was a need to increase utilization of personnel rather than simply speed up lab operations by throwing more money and people at the problem.
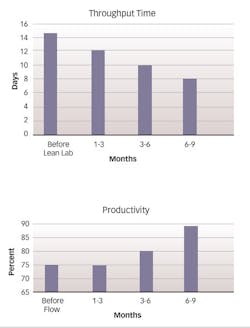
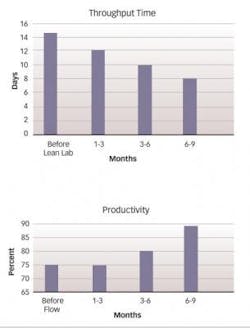
The comprehensive transformation process, of which the Lean Lab initiative was a part, resulted in significant improvements in productivity and cycle time, ultimately powering a 22% reduction in overall conversion cost (see three charts at above, right).The lab reduced testing turnaround time by more than 50%, simultaneously increasing analyst productivity by more than 25%, and took Right-First-Time from 95% to a predictable performance exceeding 99%, without any increases in the amount of equipment or personnel (see charts at the middle of next page).
Applying Lean to QC lab operations presents some unique challenges. Lean has long been practiced in manufacturing and is relatively well understood there, but its application has lagged in laboratory operations. Many examples have been published describing 5S initiatives in the laboratory, but few case studies exist, showing where manual scheduling has been eliminated in favor of “pull” sample flow.
This fact may stem from the mindset that, due to the technical complexity of testing, the QC lab is somehow “outside” the production process. Organizations that appreciate the complexity of analysis, but at the same time recognize the lab as an interdependent sub-process of the overall product conversion operation, are at a distinct advantage.
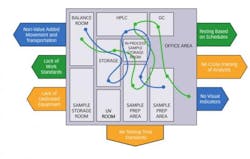
In virtually all Lean Lab initiatives, sample scheduling is recognized as a non-value-added activity and, as such, becomes a primary target for improvement. One organization with which we recently worked began its Lean Lab journey with more than a dozen scheduling tools in use. Some were formal parts of Operations Planning and MRP, but many were ad hoc worksheet based work-arounds that evolved in response to highly volatile demand patterns and constant expediting. When the company’s Lean project team quantified the amount of time spent on scheduling alone, they were astounded to find that it constituted more than 13% of the entire lab operation time.
This example clearly showed how a lack of understanding of interdependency can drive waste up and down the manufacturing cycle. In this case, the Operations Planning department did not understand laboratory capacity constraints. As a result, it was not aware that its decisions, and those of the procurement department, were creating large peaks and valleys in scheduling, which sometimes drove lab work center loading to over 100% and, at other times, left instruments and analysts idle. Operators in the lab, using its dozen scheduling “tools,” felt that they were doing all they could to push samples through the lab. As evidence, they pointed to an on-time release exceeding 95%. Hidden from everyone’s view, however, was the fact that the planning department had changed metrics. The planning team had increased sample release lead time planning values to reflect average lab performance in this chaotic environment, as opposed to setting standards that would optimize downstream manufacturing operations. As a result, even if the lab had an on-time release performance of 100%, overall manufacturing cycle time and work in process would be inflated.
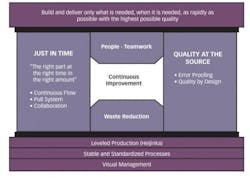
What seems to differentiate high Lean performers from less successful ones is the difference between streamlining and eliminating waste. Where the less successful aim, simply, to streamline the manual sample scheduling process, high performance organizations strive to eliminate it entirely in favor of true visual cue and “pull” systems.
They recognize that, for visual or “pull” sample management to work, a foundation of Lean prerequisites is needed, each of which contribute to improving throughput speed, capacity and predictability.
The fundamental prerequisite of establishing flow, for example, requires a holistic and coordinated approach inclusive of all Operations functional groups. To be successful, efforts must focus on establishing mechanisms that balance workload against capacity in a sustainable way. Naturally, creating capacity-balanced workloads in the laboratory requires assuring that there is sufficient capacity to meet peak demand. Where capacity is insufficient, basic Lean waste reduction tactics help increase it by increasing productivity and reducing cycle time. This is synergistic with simultaneous efforts to smooth demand peaks by reducing test cycle time variability and sample demand volatility. Obviously, everything hinges on developing a strong understanding of both internal and external factors affecting productivity and capacity. Without that, as outlined in the example above, there will be an unclear understanding of lab operations and capacity by functional groups upstream and downstream of the lab. As a result, non-value-added activities will increase, and with them, unnecessary costs in the lab and in those functional groups.
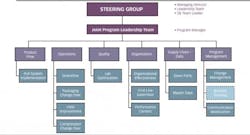
In our case study, the company-wide transformation initiative began with the creation of a team of representatives from manufacturing, QA/QC, packaging, and planning and scheduling. Each representative led sub-teams in his or her area to help design, implement and maintain the new way of working.
They used a six-step approach :
1. Identify how the lab creates value
2. Map and improve the entire value stream, not just parts of it (which only creates bottlenecks elsewhere)
3. Target waste reduction affecting throughput variability as well as speed
4. Level the laboratory’s load and mix of samples
5. Create a system that “pulls” samples rapidly through the Lab based on supply chain priorities
6. Measure performance to keep it on track
From the outset, the lab team recognized an ultimate goal of implementing visual cues to manage sample flow and set about building the prerequisite Lean foundation. The team began by analyzing incoming workload over the preceding year. The arrival patterns and mix of samples revealed significant variation on a daily, weekly and annual basis, both in terms of overall volume and the mix of sample types. The lab team assessed lab capacity, not just in terms of averages, but also in terms of their ability to manage the peaks and valleys. Because the overarching goal of the company-wide transformation was a 20% or better reduction in product conversion cost, it was understood that increasing capacity must occur through improvements in productivity, speed and predictability.
Examining their work practices through the lens of Lean thinking created a new perspective. This allowed the laboratory team to visualize their current state, identify opportunities and prioritize corrective actions.
Clear opportunities bubbled to the surface that allowed motion and wait-time waste to be reduced. To reorganize physical proximity, the team opted for cell- based work spaces for high-volume samples. While these efforts might seem to have focused on improving productivity and throughput time, they made a significant positive collateral impact on “Right-First-Time” and in creating the predictability required to reduce sample lead time planning values. Moreover, they increased “On-Time-Release” percentages in a way that was meaningful and effective for the overall manufacturing process.
- Reorganizing the placement of glassware and other supplies
- Consolidation and/or outsourcing of reagent preparation
- Kitting to reduce wasted motion
- “Hot-prep” for bullet train turnaround times
- Cell based (operating room) organization for high volume tests
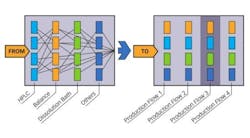
The team assessed capacity and designed what they felt would optimize the flow of test samples through the lab, including the equipment on which they would be tested and the personnel who would test them. Then, in close coordination with concurrent efforts in manufacturing and packaging, they allocated samples to dedicated test lanes. To design testing lanes for the products in the company’s solids family, for example, the team analyzed a complex set of data that included all of the factors that could affect the speed and productivity of the lab in the context of their role in the overall production cycle. Similar analysis and design work was undertaken for the five other product groups. Sample flow went from instrument-centric to product-centric. Key performance indicators were identified and visual management tools were implemented in order to maintain open communication and feedback.
Throughput time for the solids group dropped from 15 days to just eight days, an improvement of 46%, which far exceeded the target of 30% and is likely to drop to as few as six days within a year of implementation. People utilization — defined as the percentage of time that personnel spend in value-adding activities — climbed from about 65% to 90%. Within the solids group, the percentage of cross-trained personnel — that is, those who are trained to perform all tests as well as the review step — rose to 100% within two months of implementation, an increase of 42%. The percentage of Right-First-Time testing climbed from 95% to 98%, reducing laboratory investigations and decreasing laboratory throughput time.
Granted, visual “pull” sample management and understanding the interdependence of functional groups is not the only key to successful Lean Lab transformations. Nevertheless, this case study shows how important their leverage is to producing a competitive advantage. More importantly, the culture and capabilities that resulted not only produced a competitive advantage in the short term, but allowed the company to gain a significant ongoing competitive advantage. As a result of its Lean Lab efforts, it is now much better positioned to dynamically react to the inevitability of continuously changing market demand and new product introductions.